IPA Europe Session ‘The future of probiotics in Europe: A Pathway for a sustainable Growth’
The 11th edition of the International Congress “Probiotics, Prebiotics and New Foods” was again a great opportunity of exchange on the topic of probiotics from a scientific and regulatory point of view. The collaboration with the Congress resulted in a successful IPA Track day. Several angles were raised, from regulatory, scientific and consumer perspective, in Europe and worldwide.
Regulation and safety requirements are not the same, and the messages about food should not be mixed with the messages for other product groups, which is currently the case.
The IPA Track day started with the regulatory overview, with the updates from Canada, USA, Australia and Argentina authorities, followed by the IPA Europe session. The regulatory part wrapped up with a Codex overview and the work IPA initiated at the Codex Committee of Foods for Special Dietary uses for a probiotic standard.



IPA Europe President Esben Laulund presented the European landscape, he outlined approaches and responsible practices. The study of the microbiome stability is key to understand its relation to health maintenance. Some microorganisms, normally referred to as probiotics, can play an important role to support a balanced gut microbiota. This will result in considerable savings in the cost of health care systems, thanks to the impact of probiotic consumption on health and well being. Probiotics are therefore a sustainable way to maintain health and well-being. Laulund stressed the contribution that the probiotic sector can bring to the European Farm to Fork strategy.
DOWNLOAD THE PRESENTATIONThe conclusion was that there is a clear discrepancy in Europe between the existing science, the expectations of consumers that want correct information on products’ labels and the lack of a conversations on the regulatory aspects.
Rosanna Pecere presented the status of the use of the term probiotic in Europe.
Despite probiotics not being a priority for the European Commission, the use of the term is a reality today in several European countries, either with the reference to nutritional/physiological effects, or as the name of the category. Also, other countries in Europe are considering developing National Guidelines that can help to better identify food containing probiotic microorganisms.
DOWNLOAD THE PRESENTATIONThe legal expert Katia Merten-Lentz of Keller & Heckman Belgium: Probiotics? Achieving a better regulatory fit: the legal interpretation.
Merten-Lenz presented the legal reference for probiotic food and food supplements, underlining the lack of legal recognition both for probiotics as a category of food and by EFSA for the use of the term as health claim. The EC confirmed in several occasion the cut out positioning of considering probiotic as a health claim only. For EFSA, the lack of the cause-and-effect relationship is the reason of constant rejection of probiotic health claims.
DOWNLOAD THE PRESENTATIONThe impossibility to have a clear communication for the probiotic category, coupled with the various and numerous national applications, are likely to mislead the consumer, she concluded that ‘it is time for the European Union to face up to its responsibilities and harmonise the legal framework’.
From the scientific perspective, IPA Europe is also involved in the scientific debate and its experts are engaged to set clear criteria to identify probiotic microorganisms in food and food supplements. ‘The use of probiotics to correct dysbiosis of the microbiota due to disruptive events is attested by numerous studies’. Sylvie Binda, chairwoman of the IPA Europe Scientific Working Group, presented the “state of art” on microbiome resilience, which is defined as the capacity to restore the normal microbiota which can be temporary unbalanced due to stress, antibiotic, changes in the diet.
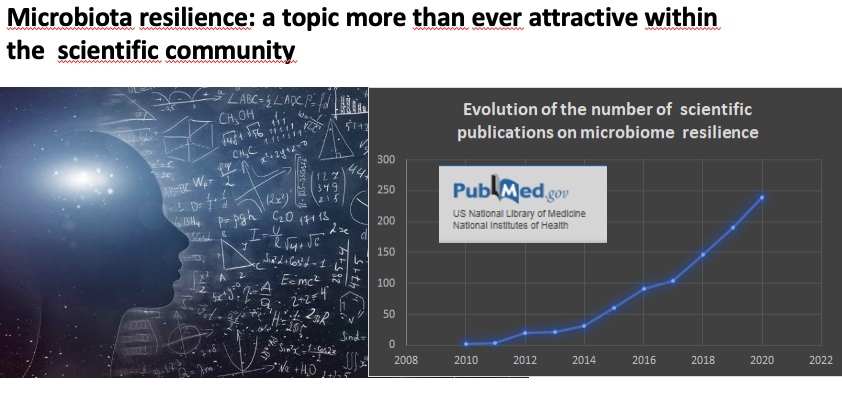
“The study of the microbiome stability is key to understand its relation to health maintenance and how probiotics can play a positive role to support microbiome resilience’ concluded Sylvie, who also invited experts to join and contribute to elucidate and build consensus.
20 Years After the FAO/WHO Guidelines: What’s Next? François Bourdichon, Food Safety Microbiologist, Chair of the Standing Committee on Microbiological Hygiene, International Dairy Federation.
DOWNLOAD THE PRESENTATIONTo conclude, science on probiotics is progressing, the political debate is also looking for a better use of the term probiotic. It appears that an engagement of the European Commission to dialogue with national authorities, in order to develop a better context for probiotics in food and food supplements, is both necessary and inevitable. This will also meet the consumers’ expectations to receive appropriate information and correct advertising.
Watch all the videos of the 11th Congress “Probiotics, Prebiotics and New Foods” in IPA Europe’s YouTube Channel.



















